고정 헤더 영역
상세 컨텐츠
본문
Thomson's atomic model was proposed in 1904 and was called the plum pudding model. It was introduced right after Thomson's 1897 discovery of the electron, then called corpuscles. He said that no matter where matter came from, it contained particles that were the same and are smaller than the atoms that matter is formed from. The model was a round thick liquidy substance whose total charge canceled out the charge of the electrons.He came to this conclusion by using a cathode ray scope. This theory was proved wrong by the gold foil experiment by Rutherford. This experiment said that the atom contained a nucleus which had a highly positive charge.

When this experiment came out, people drifted away from Thomson's theory on the atomic model and moved towards Rutherford's.
JJ Thomson: The Plum Pudding Model. 1. JJ Thomson: the PLUM PUDDING MODEL!!! Michelle Kao Elysia Hung. Plum pudding is not too bad to eat JJ Thomson’s Plum Pudding Model is not too bad to see. Index!!
Thomson Cathode Ray Tube (CRT) Cathode Rays!! And the.amazing Plum Pudding Model!! @/ Andthe Dalton’s Model . J.J. Thomson is a British Physicist and a Nobel Prize Winner who discovered electrons and isotopes in 1897. And Died in 1940.
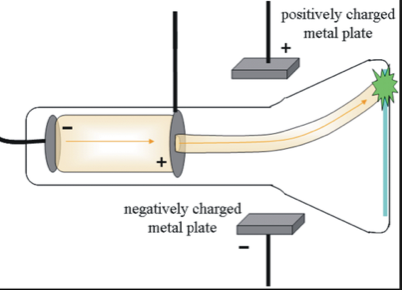
Background Information. The glow is actually many many many many many many many many many many many many many many many many many many many many many many many many many many many many many many ELECTRONS!.
Electrons can pass through the CRT because there is no air in the vacuum to stop the electrons. So that the electrons won’t bump into things. SO BEAUTIFUL. This is one pudding’s sad, short story Intermission A story about a freedom longed for, but never attained. A TV or a computer screen has many cathode ray tubes to make the different colors that appear on the screen. Same charges repel, and opposite charges attract. Magnet The ray arcs away from the magnet.
A ball of positive with little balls of negatively charged particles inside The Plum Pudding Model. Dalton’s Model Dalton believes that an atom is a solid ball. Each elements are different shaped balls since each atom has a different property. Thomson switch the cathode and anode with different metals and found out that they all create a cathode ray. He concluded that whatever is present in an atom is also in all the other atoms. (electrons). SUMMARY TIME!!
Jj Thomson George Project Function
Thomson discovered electrons in 1897. Cathode Ray Tubes are vacuums with cathode and anode. The ray arcs away when a – magnet is close.
Plum pudding model is a ball with electrons stuck in it. He disproved Dalton’s Model. Thanks for watching!




